The Ministry of Science and Technology of China recently announced a list of projects under the category of ‘Stem Cell Research and Organ Repair’ to be funded by the National Key R&D Program. A project submitted by Xu Renhe, associate dean (research) and Distinguished Professor of the Faculty of Health Sciences (FHS), University of Macau (UM), is among the projects on the list and will receive a grant of RMB 30 million. It is the first project from Macao and the first project led by UM that has been listed by the National Key R&D Program.
In 2022, a total of 27 universities and institutions have made the list under this category. They include renowned universities such as Peking University, Tsinghua University, Tongji University, and Zhejiang University. UM is the only higher education institution in Hong Kong and Macao on the list. Yonghua Song, rector of UM, hopes that the university will make unremitting efforts in contributing to the national strategic development of science and technology. According to Prof Xu, the funding of the project under the National Key R&D Program is a huge encouragement to him and his collaborators. Through this project, which is titled ‘Study of Technical Systems for the Monitoring and Assessment of the Treatment of Graft Versus Host Disease and Some Other Important Diseases with Mesenchymal Stem Cells’, they aim to facilitate the standardisation and regulation of stem cell-based therapeutic approaches to strengthen scientific collaboration and the commercialisation of research results between Macao and mainland China. He is also grateful to UM and the Science and Technology Development Fund of Macao (FDCT) for their long-standing support and assistance through the project application process.
The project (File no: 2022YFA1105000) was jointly submitted by researchers from UM, the Guangzhou Institutes of Biomedicine and Health of the Chinese Academy of Sciences, Sun Yat-sen University, Shanghai Taichu Biotechnology Company Limited, ShanghaiTech University, and Nanfang Hospital of Southern Medical University. The project was recommended by FDCT and the application was eventually approved by the Ministry of Science and Technology.
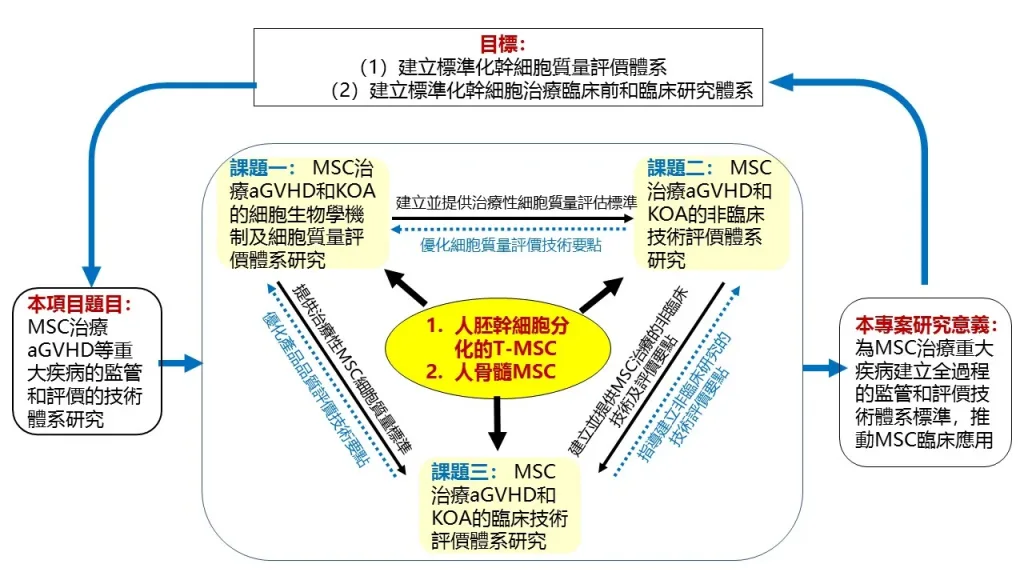
The project focuses on mesenchymal stem cells (MSCs) from different sources, including those differentiated from human pluripotent stem cells (T-MSCs) and those isolated from human bone marrow via donation, for treating disease such as graft-versus-host diseases and knee osteoarthritis, as well as establishing monitoring and assessment systems for basic research as well as clinical and non-clinical research on MSCs. The research results will help to promote the long-term and healthy development of the stem cell industry in China and increase the capability and international competitiveness of the country in stem cell therapy.
Members of the research team has accumulated rich experience in basic research, non-clinical and clinical research on MSCs, and the formulation of technical guidelines for non-clinical evaluation of cell drugs. Prof Xu has developed T-MSC together with Zhuhai Hengqin ImStem Biotechnology Company and the T-MSC has been approved by the United States Food and Drug Administration for clinical trial in the US for multiple sclerosis. Prof Xu will also cooperate with Chen Guokai, professor in the FHS, to use his cell models to study the physiological and pathological role of MSCs during disease development. Prof Xu has worked in stem cell research and therapeutic applications for many years. He has published about 90 papers in top journals, such as Nature Biotechnology, Nature Methods, Cell Stem Cell, PNAS, and Biomaterials, and they have been cited nearly 9,000 times. He has also been granted more than 10 patents in the US and China. Prof Xu is a council member of the China Society for Stem Cell Research and China Society for Cell Therapy, the founding president of the Macau Society for Stem Cell Research, and an executive editor of International Journal of Biological Sciences. Since joining UM in 2014, he has received a significant amount of funds from the university, FDCT, and the National Natural Science Foundation of China through different funding programmes.
The National Key R&D Program focuses on supporting major studies that concern social welfare and people’s livelihood, as well as strategic, fundamental, and forward-looking major scientific issues, major common key technologies and products that are related to the core competitiveness of industries, overall independent innovation capability, and national security, with the goal of providing continuous support and guidance in key areas of economic and social development. The category of ‘Stem Cell Research and Organ Repair’ under the program aims to address key scientific questions in stem cell development and organ repair. It covers the studies of basic theories and key technologies related to stem cell fate regulation, mechanisms for organ genesis and aging, organ function remodeling and manufacturing, methodologies of gene editing and regulation, and stem cell models for human diseases. It also encourages the exploration of frontier areas such as organ regeneration drugs, bio-artificial organs, and disease organoid models. The listed projects are expected to provide innovative theories and technologies for the repair and replacement of important tissues and organs and the diagnosis and treatment of major diseases.
Source: Faculty of Health Sciences
中國科技部公佈了國家重點研發計劃“幹細胞研究與器官修復”重點專項立項資助名單。澳門大學健康科學學院副院長(研究)、特聘教授兼項目首席科學家徐仁和申報的項目獲得立項,總資助金額為人民幣3千萬元。這是澳門首個獲批、澳大首次牽頭承擔的國家重點研發計劃項目。
2022年度一共有27間高校及機構獲得這個重點專項立項資助,包括北京大學、清華大學、同濟大學、浙江大學等國內多所知名高校。澳門大學是港澳地區唯一一間獲得此專項立項的高校。澳大校長宋永華期望澳大再接再厲,在國家科技發展戰略中發揮更大的作用。徐仁和表示,獲得國家重點研發計劃資助對他及他的合作者是一個很大的鼓勵,希望通過獲資助項目“間充質幹細胞治療移植物抗宿主病等重大疾病的監管和評價的技術體系研究”推進國家的幹細胞成藥的標準化和規範化,加強澳門和內地的科技合作和產業化。他十分感激澳大及澳門科學技術發展基金長期的大力支持,並在整個項目申請過程中提供協助。
該項目(檔案編號:2022YFA1105000)由澳大與中科院廣州生物醫藥與健康研究院、中山大學、上海泰楚生物科技有限公司、上海科技大學和南方醫科大學南方醫院的研究團隊聯合申報,並由澳門科學技術發展基金推薦,最終獲得中國科技部批准立項。項目聚焦不同來源的間充質幹細胞(MSC),包括由人多能幹細胞分化而成的T-MSC和從人類捐獻的骨髓MSC,治療移植物抗宿主病和膝骨關節炎等重大疾病的基礎、非臨床和臨床研究的監管和技術評價體系。項目成果將有利於中國幹細胞產業的長期健康發展,提升中國在幹細胞治療領域的水準和國際競爭力。
整個團隊在MSC 的基礎研究、非臨床和臨床研究和細胞藥物非臨床評價技術指導原則制定等方面積累了豐富的經驗。徐仁和與珠海橫琴愛姆斯坦(ImStem)生物科技公司聯合開發的T-MSC已獲美國食藥監局批准,在美國開展多發性硬化症的臨床試驗。徐仁和亦與澳大健康科學學院教授陳國凱合作,使用陳教授的多個細胞模型,研究MSC在疾病發生中的生理和病理作用。徐仁和長期從事幹細胞研究和治療應用,在Nature Biotechnology、Nature Methods、Cell Stem Cell、PNAS、Biomaterials等著名雜誌發表論文近90篇,被引用近9,000次,並獲得10多項中美專利。他亦是中國幹細胞研究協會和中國細胞治療協會理事,澳門幹細胞研究協會創始會長,《國際生物科學雜誌》(International Journal of Biological Sciences)的執行編輯。2014年加入澳大以來,徐仁和通過多個競標項目,獲得澳大、澳門科技發展基金和國家自然科學基金的大力資助。
國家重點研發計劃是針對事關國計民生的重大社會公益性研究,以及事關產業核心競爭力、整體自主創新能力和國家安全的戰略性、基礎性、前瞻性重大科學問題、重大共性關鍵技術和產品,為國民經濟和社會發展主要領域提供持續性的支撐和引領。國家重點研發計劃“幹細胞研究與器官修復” 的目標是圍繞幹細胞發育與器官修復關鍵科學問題,開展幹細胞命運調控、器官形成與衰老機理、器官功能重塑與製造、基因編輯與調控方法、人類疾病幹細胞模型等方面的基礎理論和關鍵技術研究,並開展器官再生調控藥物、生物人工器官、疾病類器官模型等前沿探索,為重要組織器官修復與替代及重大疾病診療提供創新理論和技術。
新聞來源:健康科學學院